ABOUT
OnkosXcel Therapeutics, LLC is a wholly owned subsidiary of BioXcel Therapeutics, Inc., focused on developing innovative medicines in immuno-oncology utilizing artificial intelligence. The subsidiary was formed in 2022 to advance new treatment options for patients with hard-to-treat tumors.
Investors and media: For more information on OnkosXcel Therapeutics, please visit the Investor & Media page.
Innovative Medicine Backed by the Power of Al
OnkosXcel Therapeutics applies Al to identify,
re-innovate, and develop potential new
immuno-oncology therapies aimed at
transforming patients’ lives
Growing Need for Optimal Treatment
We aim to improve the lives of the growing number of patients with aggressive cancers. In 2024, an estimated 299,010 men in the United States will be diagnosed with prostate cancer, which is classified as a “cold” tumor. Of those, an estimated 20% are expected to advance to mCRPC (metastatic castration-resistant prostate cancer), a form of advanced prostate cancer that is no longer responding to testosterone-lowering hormone treatments and has spread to other areas of the body such as the lymph nodes, bones, bladder, rectum, liver, or lungs. Approximately 80% of mCRPC cases are of the adenocarcinoma phenotype, which represent an estimated 47,842 patients.
The other 20% of mCRPC cases are of the small cell neuroendocrine phenotype (SCNC), which represent an estimated 11, 960 patients. SCNC is much more aggressive than adenocarcinoma, and represents an underserved, growing patient population, with cases increasing due to earlier and more widespread use of androgen receptor inhibitors.
Currently approved options are suboptimal for too many of these patients.
Immuno-Oncology Clinical Development
Our most advanced immuno-oncology asset, BXCL701, is an investigational oral innate immune activator being developed as a potential therapy for the treatment of aggressive forms of prostate cancer, pancreatic cancer and other solid and liquid tumors.
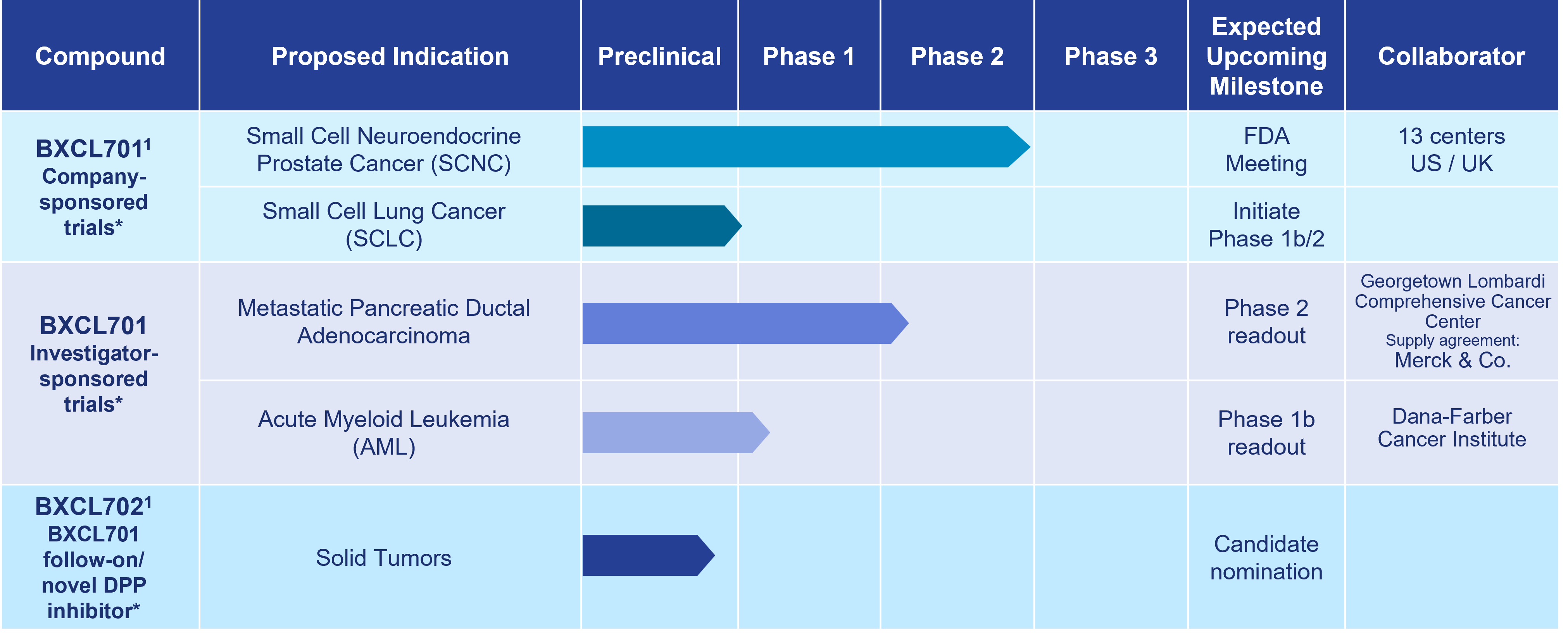
As of October 15, 2024
1 Development paused due to Strategic Reprioritization announced on August 14, 2023
*The safety and efficacy of these investigational agents have not been established.
BXCL701
Approved and experimental immunotherapies often fail to address cancers that appear “cold.” Therefore, BXCL701 is being evaluated to determine if it can render “cold” tumors “hot,” making them more detectable by the adaptive immune system and thereby facilitating the development of a strong anti-cancer immune response. OnkosXcel Therapeutics’ preclinical data support BXCL701’s potential synergy with currently approved checkpoint inhibitors and emerging immuno-therapies directed to activate T cells.
BXCL701 completed a Phase 2 clinical trial in combination with pembrolizumab in mCRPC, SCNC,
and adenocarcinoma. Updated Phase 2 clinical trial data were presented at the Prostate Cancer Foundation Annual Scientific Retreat in November 2023.
BXCL701 has received Orphan Drug Designation from the U.S. Food & Drug Administration (FDA) in four indications: acute myelogenous leukemia, pancreatic cancer, stage IIb to IV melanoma, and soft tissue sarcoma. BXCL701 has also received Fast Track designation from the FDA for SCNC. An 800+ subject clinical database with data collected by the Company and others supports the ongoing development of BXCL701.
The safety and efficacy of BXCL701 have not been established.
Clinical Trials
Learn more about the BXCL701 Phase 2 clinical trial (NCT03910660) and trials with other investigational drugs here.

